semiconductor materials
Semiconductor materials have made up of two adjacent pieces of p-type and n-type semiconducting materials. P-type and n-type materials are simply semiconductors, such as silicon (Si) or germanium (GE), with atomic impurities; the type of impurity present determines the type of the semiconductor. The process of purposefully adding impurities to materials have called doping; semiconductors with impurities have referred to as “doped semiconductors“.
P-TYPE P Semiconductor Materials
In a pure (intrinsic) Si or GE semiconductor, each nucleus uses its four valence electrons to form four covalent bonds with its neighbors (see figure below). Each ionic core, consisting of the nucleus and non-valence electrons, has a net charge of +4 and have surrounded by 4 valence electrons. Since there are no excess electrons or holes In this case, the number of electrons and holes present at any given time will always be equal.
Now, if one of the atoms in the semiconductor lattice has replaced by an element with three valence electrons, such as a Group 3 element like Boron (B) or Gallium (Ga), the electron-hole balance will be changed. This impurity will only be able to contribute three valence electrons to the lattice, therefore leaving one excess hole (see figure below). Since holes will “accept” free electrons, a Group 3 impurity has also called an acceptor.
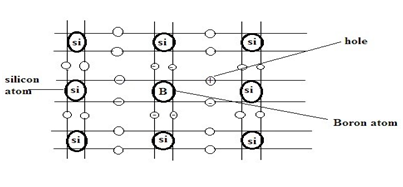
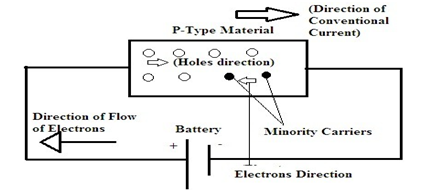
Because an acceptor donates excess holes, which have considered to be positively charged, a semiconductor that has been doped with an acceptor is called a p-type semiconductor; “p” stands for positive. Notice that the material as a whole remains electrically neutral. In a p-type semiconductor, the current has largely carried by the holes, which outnumber the free electrons. In this case, the holes are the majority carriers, while the electrons are the minority carriers.
Silicon Doped with Boron
In this way, the semiconductors that are rich in holes as there carriers formed by the trivalent impurities comes under the list of p-type semiconductors.
Whether silicon or germanium if they have added with a trivalent impurity that belongs to p-type of the semiconductor.
P-type and N-type semiconductor materials
When the external supply of voltage has given to the p-type semiconductor their majority of carriers present in the valence band tend to move towards the negative terminal of the supply and the minority carriers that are electrons present in the conduction band move towards the positive terminal.
N-TYPE Semiconductor Materials
In addition to replacing one of the lattice atoms with a Group 3 atom. We can also replace it with an atom with five valence electrons. Such as the Group 5 atoms arsenic (As) or phosphorus (P). In this case, the impurity adds five valence electrons to the lattice where it can only hold four. This means that there is now one excess electron in the lattice (see figure below). Because it donates an electron, a Group 5 impurity has called a donor. Note that the material remains electrically neutral.
Donor impurities donate negatively charged electrons to the lattice. So a semiconductor that has been doped with a donor is called an n-type semiconductor; “n” stands for negative. Free electrons outnumber holes in n-type material, so the electrons are the majority carriers and holes are the minority carriers.